Food safety management tools
This part is primarily in three sections (click to jump to topic); HACCP, microbiological criteria and microbiological risk assessment. But first I've written a prolog with links to reports covering the current and future issues in food safety control:
- Guidelines for developing harmonised food control systems
- "Terrorist Threats to Food: Guidance for Establishing and Strengthening Prevention and Response Systems" from the WHO (pdf)
- FDA registration of food facilities; response to bioterrorism threat
- Risk Assessment for food terrorism and other food safety concerns
- FAO - Food quality & Safety
- IFT report - Food Safety issues for the 21st Century
- CAST recommendations
- CAST food safety
- American Academy of Microbiology - food safety, current and future needs
- Control of Listeria in small meat and poultry plants
- Guidelines for developing GMP, SOP, and environmental sampling plans for RTE products
- Listeria monitoring program
- Chlorination
Something to consider concerning food safety implementation and practice
A food processing unit supplies an exhibition arena used for major events such a conferences and music concerts. Therefore it has multiple menus, large numbers of consumers who (presumably) consume the food within hours of purchase. The hygiene rules of the company are:
- Reject if chilled products are >5oC
- Reject of frozen products >-18oC
- Reject any dented, blown or damaged tins
- Keep cold food cold (<5oC)
- Keep hot hoot hot (>63oC)
- Keep prepared food out of the Temperature Danger Zone (5-63oC)
- If any refrigeration unit fails and the chilled food temperature is above 8oC then it must be used, reprocessed or discarded within 4 hours.
Consider the following questions:
- Are the rules achievable?
- What is the significance of the set temperatures, ie -18, 5 and 63oC ?
- What organisms could grow to significant numbers at temperature above 8oC.
- If the legal limit for chilled foods is 8oC, then why is the company using 5oC?
- Despite these 'in-house' temperature regulations, which organism(s) could nevertheless cause a major food poisoning outbreak from this establishment?
HACCP
HACCP is the abbreviation for 'Hazard Analysis Critical Control Point'. It is the internationally accepted means of safe food production. The essential aspects are that hazard are identified and control measures implemented as opposed to the previous retrospect approach of reliance upon end-product testing. The latter being inadequate as a controlling method since the results (especially from microbiological analysis) would not be obtained until after the food had been packaged and distributed.
You can down load a Powerpoint presentation I've written on HACCP, MRA and microbial criteria by clicking here.
It should be remembered that hazards in HACCP include physical and chemical hazards, not just microbiological. Also in the next edition I plan to expand on food additives and materials in contact with food since I am on the EU Food Safety Authority panel which considers these compounds. This includes the evaluation of semicarbazide in jar baby foods (chemical hazard: HACCP) which has caused concern in the Summer of 2003.
Before HACCP can be properly implemented there are a number of 'Prerequisite Programmes' or 'PRP' that are required. These have been defined by the WHO (1999) as 'Practices and conditions needed prior to and during the implementation of HACCP and which are essential for food safety, as described in Codex Alimentarius Commission's General Principles of Food Hygiene and other Codes of Practice.''.
- Building design and work flow pattern
- Equipment design
- Supplier control
- Receiving, storage and distribution
- Specifications
- Staff training and personal hygiene
- Cleaning and disinfection
- Pest control
- Chemical control
- Traceability and recall
- Waste management
This list is largely taken from the NACMCF 1997 HACCP System, and many of these are covered in more detail in my book with Pat Hayes 'Food Hygiene, Microbiology and HACCP' (originally published by Aspen, now Kluwer Academic) which was written for industry rather than undergraduates.
- Conduct a hazard analysis (HA)
- Determine the critical control points (CCPs)
- Establish critical limits
- Establish monitoring procedures
- Establish corrective actions
- Establish verification procedures
- Establish documentation and record-keeping procedures
A 'hazard' may be biological, chemical or physical. Although the book primarily deals with microbial hazards, there are many others such as glass, cleaning agents and pesticides. There has been a lot of attention is on acrylamide. Here are two web sites relevant to the FDA and EU (pdf file) stance on this compound. There has also been a well publicised 'food poisoning' in Nanjing (China) that was due to food being contaminated with rat poison. However the most severe is probably the deliberate use of melamine in infant formula. There are numerous news sites on this issue and the appalling consequences to infant health (URL). The topic is referred to on page 13.
The legal aspect of HACCP implementation is complicated and varies from country to country. Until June (2002) in the UK the first 5 principles were the only legal requirement. Large food companies would have Principles 6 (Verification) and 7 (Documentation and Record-keeping) in place. Smaller companies, although not obliged to have the latter principles, would be recommended to adopt them as a support for 'Due Diligence' in a court of law. You may wish to consider how efficient a food company can control its hazards without documentation or verification - and I'd be interested if you could prove the saying "If it's not written down, it doesn't exist" wrong! This situation changed on 7th June (2002) with the Food Standards Agency (UK) announcement that meat and poultry plants required the full 7 stages. Additionally there are now draft proposals concerning consolidating and simplification of the EU food hygiene legislation. Essentially the proposals would require all non-primary food operators to implement a harmonised HACCP system, records to be kept of food safety checks carried out under HACCP, codes of good practice to be used as the food safety management on farms (instead of full HACCP implementation) and the compulsory registration of all food businesses. The two pdf files concerning these proposals can be down loaded here (1) and (2) .
Another question you consider is "'How can small food companies identify the hazards appropriate to their food production? Where will their expertise come from?". The reason for the question is that there has been a problem in the past in that certain regulatory authorities have not assisted in HACCP-like implementation in case the system breaks down and they are blamed. Fortunately there are numerous independent experts available. But a key aspects is that HACCP implementation cannot be an off-the-shelf software package, it requires representatives from the factory floor to state what really happens during production as opposed to what management think happens. In 2002 HACCP implementation for companies handling meat was released by the HMSO for Northern Ireland and includes swabbing sites and microbiological levels.
It should be remembered that HACCP does not only refer to microbiological pathogens, but to physical and chemical hazards as well.
My first book 'Food hygiene, microbiology and HACCP' published by Aspen (now Kluwer Academic) was focused on the complete industrial implementation of HACCP. You can also visit various HACCP sites at:
- NACMCF HACCP Principles
- Generic HACCP models from USDA/FSIS
- Generic HACCP models (USA)
- HACCP video
- HACCP resources (USDA)
- Generic HACCP models (Canada)
- Generic plan for beef jerky (Canada)
- template HACCP models for the meat industry (New Zealand)
- General HACCP models for various foods
- Generic seafood HACCP plans
- HACCP95
- HACCP and Total Quality Management
- International HACCP Alliance
Microbiological criteria
I've found a few WWW sites which summarise microbiological risk and criteria.
- Database of microbiological specifications
- World Health Organization
- Microbiological criteria in the EU
Operating characteristic curve
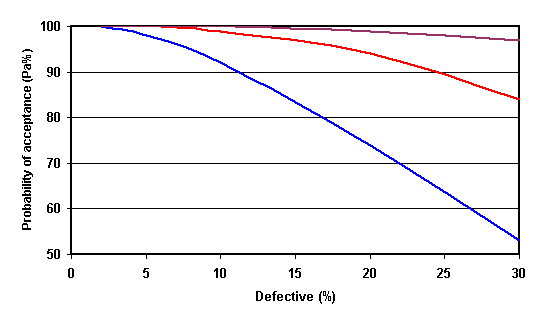
The above graph is the 'Operating characteristic curve for n=5, c= 1 to 3 (Fig 8.5a in the book). It illustrates that although a batch of food may be 30% defective there is a 52, 85 or 98% chance of accepting it using a sampling plan of 5 samples with a reject batch criteria (c) of 1, 2 or 3 respectively.
This clearly illustrates that end-product testing cannot guarantee a safe product ! Hence the need for HACCP and more recently microbiological risk assessment.HPA Ready to eat guidelines
Since the publication of the first edition of my book in 2000 the HPA (UK) guidelines for ready-to-eat foods (Table 6.14, p287) have been modified twice. The latest 2009 being as the book was being complied. Such is the pace of food microbiology and the need for web page up-dates! The 2000 version changed the 'Aerobic plate count' to 'Aerobic colony count' and the Clostridium perfringens Satisfactory level became 20/g. The units for the Table should be per gram unless otherwise stated, ie per 25 g as for Salmonella etc.
The new 2009 HPA ready-to-eat guidelines can be downloaded as a .pdf file version by going to the HPA (UK) web site link.
In 2002 HACCP implementation for companies handling meat was released by the HMSO for Northern Ireland and includes swabbing sites and microbiological criteria.
Microbiological Risk Assessment (MRA)
Below are over 70 MRA sites to visit to keep you busy...!
Spreadsheet RA model
Ross and Sumner have published two papers in International Journal of Food Microbiology (vol77, 39-53 and vol77, 55-59; 2002) on risk assessment tools. The first paper is on a simple, spreadsheet which can be down loaded (ZIP file based on ExcelTM). It includes two case studies; viruses in oysters and comparison of risk estimate with Cassin et al. (1998).
Salmonella
-
FAO-WHO Salmonella in eggs and broiler chickens (ftp site; two WORD files to down load) Jan 2003
- USA Elimination of S. Enteritidis illness due to eggs
- EA eggs (pdf file)
- HA broilers & eggs (pdf file)
- FSIS eggs and egg products
- Denmark, contribution of animal sources
- HI & HC Salmonella in broilers & eggs (Fazil et al pdf file.)
- EA S. Enteritidis in eggs (Ebel et al pdf file.)
- EA Salmonella in broilers (Kelly et al. pdf file)
- FSIS Salmonella in shell eggs risk assessment
- S. Enteritidis risk assessment model
- Global Salmonella surveillance (Global Salm-Surv)
Listeria
- FSIS draft L. monocytogenes RTE meat & poultry, March 2003
- FDA draft L. monocytogenes QMRA
- L. monocytogenes (Buchanan & Lindqvist, pdf file)
- Second site for FDA draft Listeria monocytogenes QMRA
- Preliminary study, health impact
- EU 1999 (pdf file)
Campylobacter
- HA, EA, HC in broiler chickens, FAO-WHO
- fluoroquinolone resistant Campylobacter
- Revised Risk Assessment for fluoroquinolone-resistant campylobacter in chickens
- Broilers Denmark QMRA
- Fazil chicken study course
Other micro-organisms and foods
- QMRA - cross-contamination
- Risk Assessment for food terrorism and other food safety concerns
- E. coli O157 in beef tartare, RIVM, Netherlands
- E. coli O157 in beef risk assessment
- Draft FSIS E coli O157 Risk Assessment (pdf file)
- V. parahaemolyticus - shellfish
- HA, EA, HC, for Vibrio spp. in seafoods
- EU Norwalk-like viruses (pdf file)
- Salads
- Lap-mei
- Siu-mei and Lo-mei
FAO/WHO
- Risk Assessment: Food Bioterrorism and other food safety concerns
- JEFRA homepage
- Principles and guidelines for incorporating MRA in the development of food safety standards, guidelines and related texts; Joint FAO/WHO March 2002 (pdf file)
- Codex Principles for MRA (pdf file)
- WHO Background paper
- JEMRA (pdf file)
- WHO Microbiological Risk Analysis
- FAO/WHO (pdf)
- CCFH response to Codex
- Joint FAO & WHO (1999) Consultation report
- FAO/WHO HC food and water (pdf)
- Joint Expert Committee on Food Additives
- antimicrobial agents, new animal drugs
- Abridged FOA MRA explanation page
- JEMRA food and water (pdf file)
- FAO/WHO food and water
- FAO/WHO assessors and managers
- Risk Analysis, Geneva 1995
- Risk Management & Food Safety, Rome 1997
- Risk Communication, Rome,1998
- Risk Assessment, Geneva, 1999 (pdf file)
- Risk Management 2000
- General - Food Control
- L. monocytogenes (Buchanan & Lindqvist, pdf file)
- HI & HC Salmonella in broilers & eggs (Fazil et al pdf file.)
- EA S. Enteritidis in eggs (Ebel et al pdf file.)
- EA Salmonella in broilers (Kelly et al. pdf file)
- Hazard characterization for pathogens in food and water
- Assessors & managers of microbiological hazards
- WHO antimicrobial resistance
- WHO index
- WHO microbiological risk assessment
EU
- Risk assessment of food borne bacterial pathogens: Quantitative methodology relevant for human exposure assessment (Final report 2003, pdf format)
- Codex 2000, Risk Analysis
- EU MRA & 93/43/EEC
- EU harmonisation
- Quantitative methodology - preliminary report (pdf)
General food & water
- Hazard Characterization of pathogens in food and water: Guidelines. JEFRA 2003
- 1996 Summary paper by Society for Risk Analysis
- Dept. of Health (UK) Advisory Committee on dangerous pathogens
- HACCP & MRA
- ILSI principles (1998)
- ILSI principles (2000; pdf file)
- International Life Sciences Institute (ILSI)
- Food Safety Risk Analysis Clearinghouse
- Risk assessment tools
- Risk assessment frameworks
- RiskWorld
- Society for Risk Analysis
- USDA Risk analysis bibliography
Online training courses and model explanations
- Risk Analysis Background from FSIS
- WHO
- FSIS E. coli O157:H7
- Poultry FARM (Oscar)
- FAO/WHO initiative on Microbial risk assessment
